Heating Cooling Curves Phase Diagrams

Heating And Cooling Curves вђ Overview Examples Expii Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. Learn how to plot and interpret heating and cooling curves, which show the phase changes of a substance when heat is added or removed at a constant rate. see examples of heating ice to steam, superheating and supercooling, and the enthalpies of fusion and vaporization.
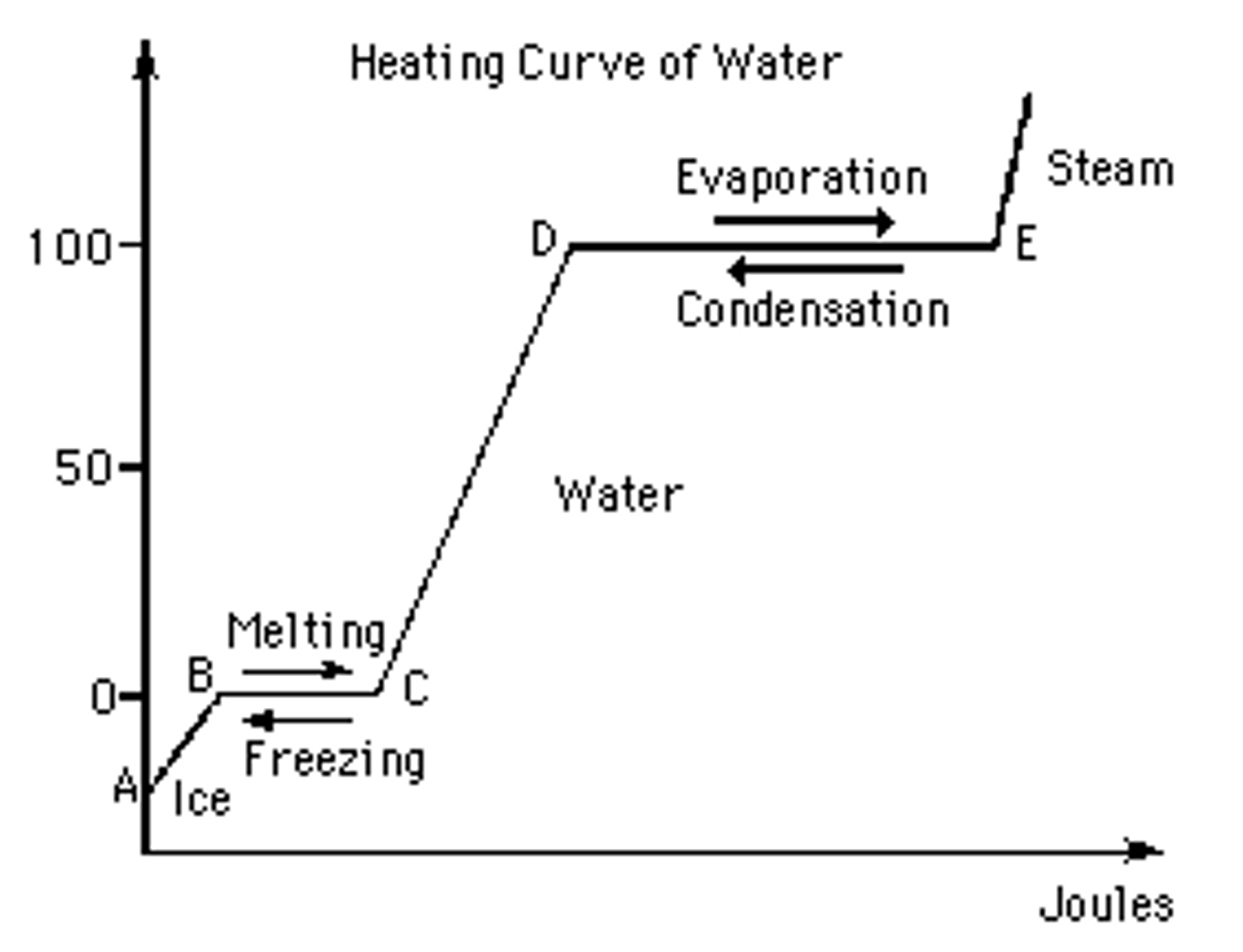
Heating And Cooling Curves Phase Diagrams At Beatrice Smart Blog Figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. the change of state behavior of all substances can be represented with a heating curve of this type. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. explore the relationship between enthalpy of vaporization and temperature and pressure with examples and exercises. The compound cholesteryl benzoate is a rod like molecule that undergoes a phase change from the solid to the liq uid crystal phase at 145.5 °c. when cholesteryl benzoate is mixed with cholesteryl oleyl carbonate, a molecule with a curved shape, the temperature of the solid to liquid crys tal transition changes. Key concepts and summary. phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase.

Heating And Cooling Curves Ck 12 Foundation The compound cholesteryl benzoate is a rod like molecule that undergoes a phase change from the solid to the liq uid crystal phase at 145.5 °c. when cholesteryl benzoate is mixed with cholesteryl oleyl carbonate, a molecule with a curved shape, the temperature of the solid to liquid crys tal transition changes. Key concepts and summary. phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase. Below is a cooling curve for water for the temperature range 115°c down to 15.0°c. for this temperature range Δh is equal to 834.0 kj. when a liquid is cooled it is an exothermic process. worksheet: heating and cooling curves part 1 worksheet: heating and cooling curves part 2. exercises. exercise 1. Heating and cooling curves. when a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and phase of the substance is observed; this is graphically represented by heating and cooling curves. for instance, the addition of heat raises the temperature of a solid; the amount of heat absorbed.

Heating And Cooling Curves Below is a cooling curve for water for the temperature range 115°c down to 15.0°c. for this temperature range Δh is equal to 834.0 kj. when a liquid is cooled it is an exothermic process. worksheet: heating and cooling curves part 1 worksheet: heating and cooling curves part 2. exercises. exercise 1. Heating and cooling curves. when a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and phase of the substance is observed; this is graphically represented by heating and cooling curves. for instance, the addition of heat raises the temperature of a solid; the amount of heat absorbed.

Comments are closed.